vendredi 10 juin 2016
PHASE EXPLOSION INJECTION
lundi 15 décembre 2008
ULTIMATE LIQUID WASTE NUCLEAR DECONTAMINATION
DECONTAMINER DEFINITIVEMENT LES REJETS LIQUIDES RADIOACTIFS
Je préconise l’association d’ozone et de désinfectants chimiques appliqués à de grands bassins d’eaux susceptibles d’être contaminées, avant rejet dans l’environnement. Nous allons voir pourquoi.
On peut dire de L'OZONE qu'il est:
- Le composé le plus oxydant après le fluor, dû à sa facilité à capter les électrons
- De décomposition facile
- De plus, dans des conditions équivalentes, il est plus stable dans l'eau que dans l'air.
Les avantages liés à l'utilisation sont nombreux:
- L'ozone élimine une grande quantité de substances préjudiciables, qu'ils oxydent comme le fer ou le manganèse, l’Uranium… décomposant les détergents, les pesticides, les herbicides, les trihalométhanes et neutralisant le cyanure, l'ammoniac, les nitrites, l'urée, etc.
- Il possède une efficacité beaucoup plus importante par rapport aux autres espèces désinfectantes, provoquant l'élimination et l'inactivation des virus, bactéries, champignons, spores, algues et protozoaires.
- Il élimine tout type d'odeurs et de couleurs dans l'eau.
- Il augmente la transparence de l'eau et le rendement des filtres puisqu'il agit comme un floculant.
- Pour le traitement des piscines, l'ozone est le plus efficace connu. En plus d'améliorer substantiellement la qualité de désinfection par rapport à une piscine traitée avec du chlore, il évite de nombreux problèmes que ce traitement traditionnel implique: typique odeur de piscine due à la formation de chloramines, irritations, gênes, asthme, maillots de bain déteints, etc.
Utilisations de l'ozone :
L'ozone peut être employé dans le traitement de l'eau avec comme objectifs :
+La décontamination nucléaire, par oxydation des métaux et alcalino-terreux et de leurs complexes organiques, jusqu’à les rendre insolubles à pH neutre; c’est le cas de l’Uranium.
+La floculation des colloïdes fins : Les colloïdes fins responsables du résiduel de contamination des eaux, sont composés par des particules entre 0.001 microns (µ) et 30 µ. La petite taille de ces particules leur permet de rester en suspension et d'échapper à la plupart des méthodes mécaniques de filtration. L'ozone permet de supprimer ces colloïdes en les regroupant (ozofloculation), ce qui permet leur évacuation par la filtration et la sédimentation.
+ La suppression des composés organiques dissous : Les composés organiques dissous (Cod) ou les produits organiques donnent à l'eau une nuance couleur thé caractéristique. Les Cod sont non-biodégradables et s'accumulent en fonction du renouvellement de l'eau. Ces Cod réduisent l'efficacité de nitrification du filtre biologique.
+L’élimination des nitrites : L'ozone élimine les nitrites par oxydation directe en nitrates réduisant la masse organique ce qui améliore l'efficacité et la nitrification dans le filtre biologique.
+La désinfection : L’ozone peut efficacement inactiver les microbes pathogènes bactériens, viraux, fongiques et les protozoaires parasites.
Purification de l'eau potable
L’ozone est employé dans le traitement de l’eau pour plusieurs fonctions :
§ oxydation des métaux;
§ amélioration de la performance de filtres à sable ;
§ amélioration de la floculation (appelée "ozofloculation") ;
§ désinfection de l’eau (attention toutefois au risque de contamination par les parasites cryptosporidium);
§ élimination de composés organiques nocifs, en particuliers pesticides et herbicides. Pour cette application l’ozone est en général injecté en amont d’un filtre à charbon actif.
L’ozone est utilisé dans des procédés de traitement des eaux usées, en particulier pour rendre digestible par des bactéries la DCO dite "dure", et pour la désinfection de l’eau en sortie de stations d’épuration (traitement dit tertiaire). Les colloïdes fins responsables de la contamination des eaux, sont composés par des particules dont la petite taille leur permet de rester en suspension et d'échapper à la plupart des méthodes mécaniques de filtration. L'ozone permet de supprimer ces colloïdes en les regroupant, ce qui permet leur évacuation par la filtration et la sédimentation.
Ces applications nécessitent la maîtrise de plusieurs technologies : ozonisation, filtration, et éventuellement bio-réacteurs.
Caractéristiques du système :
La conception du réacteur d'ozone est très importante pour la réussite et la sécurité de l'ozonation. Il y a une gamme disponible de réacteurs qui utilisent différentes conceptions (diffuseurs fins de bulles, turbine, injecteurs, colonnes, mélangeurs statiques, chambre de contact...) pour transférer l'ozone à l'eau. Pour choisir un réacteur il faut tenir compte de :
§ L'efficacité de transfert de l'ozone ;
§ L'étanchéité de la conception et construction ;
§ La construction avec les matériaux résistants à l'ozone.
L'utilisation de matériaux inadéquats peut conduire à l'érosion de l'unité de production et causer des fuites dangereuses et coûteuses. De tels systèmes ne sont pas appropriés pour une production d'ozone régulière ou à long terme. La génération de l'ozone dans des systèmes équipés avec des matériaux de qualité inférieure est également moins efficace car l'ozone est perdu pendant que les matériaux du réacteur sont oxydés.
Régimes de traitement :
L'ozone peut être appliqué sans interruption, comme traitement permanent ou simplement quelques heures par jour.
Si la décontamination est le but primaire de l'ozonation, la quantité d'ozone nécessaire dépend en grande partie du chargement en ions métalliques et alcalino-terreux de l'eau à traiter. Dans l'eau pure, une concentration résiduelle de 0.01 à 0.1 ppm pendant des périodes aussi courtes que 1 heure peuvent être efficaces. Cependant, dans l'eau en présence de charges organiques plus élevées, la période résiduelle de concentration et/ou de contact en ozone doit être augmentée pour produire une décontamination significative. Les eaux normales exigent généralement des concentrations résiduelles en ozone entre 0.1 à 0.2 ppm et des périodes de contact entre 1 à 5 heures pour la décontamination.
Emplacement de l'appareil
Il y a plusieurs endroits dans un bassin où l'ozone peut être ajouté en fonction des résultats attendus.
La mesure de l'ozone dans le bassin :
La mesure directe de l'ozone dans un échantillon d'eau est généralement réalisée en utilisant les kits colorimétriques et la spectrophotométrie d'essai. Il est préférable d'utiliser des sondes mesurant le potentiel d’oxydo-reduction (ORP). En gardant les mesures d'ORP dans une certaine marge, on peut contrôler les niveaux des oxydants.
Les risques : L'ozone est un agent d'oxydation très efficace dans le cadre du traitement à l'eau et de la réduction des charges de microbes pathogènes du bassin. Cependant, l'utilisation de l'ozone comme n'importe quel autre produit chimique de cette nature est accompagnée de risques considérables:
· La réduction par l'ozone du niveau des nitrites comporte un risque. Le filtre biologique reçoit moins de nitrite et la population des bactéries responsables du traitement des nitrites en nitrates diminue (les bactéries nitrobacter ont besoin d'un taux de nitrites de 2,5 mg/l pour pouvoir se développer). Si n'importe quelle rupture de l'ozonation se produit, une variation dangereuse de la concentration en nitrite peut survenir plus tard.
· Les concentrations résiduelles élevées d'ozone sont un risque pour la faune pouvant causer une mortalité ou des dommages (lésions) sur les tissus. Elles sont aussi un risque pour le biofilm (films bactériens) des masses de filtration biologique. La rupture du fonctionnement de la filtration biologique peut engendrer de grandes fluctuations dans les niveaux d'ammoniac et de nitrite. Ceci peut avoir un effet mortel sur la faune aquatique ou pour le moins des effets sur la santé et la croissance des poissons.
L'ozone est extrêmement toxique et l'exposition de l'homme constitue un risque sanitaire sérieux. Diminution des capacités pulmonaires, inflammation des tissus, aggravation de l'asthme, irritation de la gorge, toux... sont des symptômes typiques d'exposition à l'ozone. Dans les cas d'exposition prolongée ou grave, les maladies respiratoires chroniques telles que l'emphysème, la bronchite chronique et le vieillissement prématuré des poumons peuvent se produire. Les normes d'exposition (diverses administrations internationales de salubrité professionnelle et de sécurité) pour l'ozone résiduel s'étendent entre 0.05 et 0.1 ppm pendant une période de 8 heures et un contact maximum de 0.3 ppm pendant moins de 10 minutes.
Il est donc important d'insister sur la qualité des composants, l'étanchéité du réacteur et l'installation de production d'ozone. La mise à l'air libre des abris techniques dans lesquels sont installées les unités de production est également fortement recommandée (ventilation haute et basse). Les humains peuvent détecter les niveaux bas de l'ozone résiduel grâce à son odeur pointue et piquante, mais une exposition continue à l'ozone devient indétectable à cause de la perturbation des sens. Pour cette raison, il ne faut jamais se reposer sur la simple perception de l'odeur comme moyen d'indication de la présence d'ozone.
Des systèmes portatifs de détection de l'ozone sont disponibles dans le commerce et sont un outil utile pour aider à assurer la sûreté de personnes intervenantes dans le local technique ou près du bassin.
Il est de ma compétence d’installer et de tester un prototype simple de décontamination d’eaux faiblement chargées selon les principes exposés ci-dessus,
JR COSTES, Ingénieur Chimiste & Nucléaire
Tél 06 74 36 91 85
Email : omogine@yahoo.fr
PS : On pourra se reporter avec profit, à ma publication sur la décontamination d’échangeurs en acier inoxydable, par des mousses au Ce, repompé à l’ozone.
lundi 14 janvier 2008
REMOTE DISMANTLING OPERATIONS -WAGR DECOMMISSIONING
Exploring Innovative Approaches 2002
JR COSTES, Ch. LEGOALLER,: CEA/UDINMarcoule – France
P. VALENTIN: CEA/CEREM Pierrelatte; G. PILOT: CEA/IPSN Saclay
The WAGR (Windscale Advanced Gas Reactor) dismantling, bridgehead of future dismantlings of graphite-gas reactors, is an extremely important textbook case, which rightly should use the most modern techniques, enabling the choice of the scenario which is best-suited to lowering the doses received by the operators, the costs and the volume of the wastes.
Looking toward the future, the European Community provided precious assistance (Contract Number FI4D-CT95-0006 )
which helped to successfully implement four promising techniques on a major site:
3-D simulation of remote-operation work, is vital for:
- operators training
- specifying the machines and the tasks they must accomplish
- optimizing the cutting.
This work, which was conducted with the ROBCAD software package, was finally converted to IGRIP upon request from the Prime Contractor. The entire environment and the machines were simulated, as were the first steps of the dismantling operations.
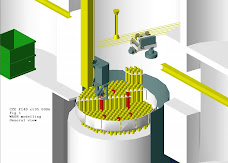
The Remote Dismantling Machine was modelled, i.e. the mast, the manipulator hoist platform, the manipulator arm and gripper, the 3 Te hoist and the maintenance and sentencing cells.
The reactor core has also been modelled including the hot box, loop tubes and the top sections of the pressure vessel (see figure 1)
Setting up the man/machine interface:
Significant CAD modelling progress has been made with the building of a Control Panel replicating the actual WAGR RDM Panel, including 4 joysticks and switches that control the rotating floor shield, the transfer hoist slew beam and the 2 hoist transfer movements (see figure 2). Additionally most of the detailed comprehensive dismantling tasks for the removal of the hot-box using RDM, have been included in the 3D CAD modelling application.
The acoustic declogging of the electro filters:
ESP filters could be rapidly be overloaded with the abundant and very fine aerosols produced by the thermal cutting. For these reasons, a method applicable in situ without removal of the filter elements and without liquid waste production was developed: acoustic cleaning ( fig 3).
Waves produced by the vibration of a membrane displace the air in which they travel: this is acoustic energy. This energy has two main parameters: the fundamental frequency measured in Hertz and its intensity measured in decibels (dB).
Compressed air is used to vibrate the membrane of the used horn, producing sufficient acoustic energy to break the adhesive bonds between the particles and the collection surfaces and thus cleaning the electrostatic precipitator.
Thanks to the addition of silencers placed in a ring around the acoustic horn, a very acceptable sound level was reached. This device is efficient and very easy to use. The horn is compressed air driven and the supply is controlled by a solenoid valve mounted in the supply line pipework.
First trial in WAGR
In February 1998, first trials on the effectiveness of the acoustic horn have been completed although the efficiency of the declogging of the ESP internal structure was not easy because the ionizer and the collector were rather clean. However, after pulses of 1 second, the radiation dose rate measurements in contact with the collection pot showed an increase from 28 µSvh-1 to 50 µSvh-1 and up to 70 µSvh-1 with 5 more pulses of 1 second due to a removal of a small amount of particulate probably from the ESP internal walls.
The noise due to the horn was heard by a man placed at the floor just above but not by a man whose position was higher (with two floors of difference).
Anticipated use for hot-box cutting, campaign 3, was considered.
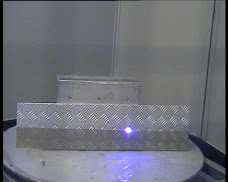
The ultra-violet laser cleaning, which enables remote removal (2 to 6 meters) of the smearable and/or fixed contamination.
The optical equipment called LECDIN enables the laser to be focussed to a spot of 25 mm wide by 2 mm high at a distance of 2 m, which uniformly scans an area 1.5 m by 1.5 m. Each shot has 350 mJ of energy, 306nm, 12 Mw of power and a repetition rate of 150 Hz.
A He-Ne red laser beam, coaxial to the UV beam, enables remote focusing of the laser impact. A camera built into the LECDIN optics locates the red pointer. The zone to be treated is defined using a beam control joystick. The computer (PC) does the rest .
During two 2-day campaigns, the instrumentation was received and tested.
On 23 and 24 June 1999, we proceeded with real shots on the following :
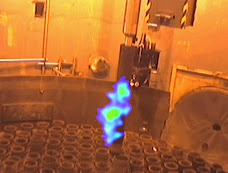
-a cylindrical grab, with fixed contamination set at 200 c/s/cm² (figure 4). It is set flat on the turntable and is treated on both sides by 180° rotation of the turntable. Large streams of violet light stream spurt out from the flush impacts, as shown by the figure. The dull surface of this stainless steel object shines like new after the treatment, some hot points at 10-20 c/s/cm² remain, but the treatment is not continued.
-on 23 and 24 February 2000, a second campaign was launched. This time corrugated sheet aluminium is proposed for decontamination. Its contamination was also set at 200 c/s/cm².
As in the preceding case, the dull alumina is removed by the laser scanning, and a shiny surface appears. The unevenness themselves are cleaned well (figure 5). After thorough treatment, then a second partial treatment, the object is left at less than 20 c/s/cm² in spots. No contamination is observed in the turntable premises.
Here again, tests show the machine's high efficiency, which does not cause any thermal effect and returns the surface to its original condition. Even painted objects can be treated without significant paint stripping
The LECDIN device would consist mainly of a powerful Exciplex laser and a penetration through the concrete wall opposite the RDM, as laser beam positioning and control provisions. This type of laser does not involve any thermal phenomena and therefore presents no danger.
The Video Gamma Camera, allows remote detection of any slightly irradiating object.
With this type of tool, on can control the cutting, movement and management of any irradiating object. This enables to improve the work scenario, insofar as the irradiating objects can be removed by remote operations, the remainder of the structure being cut manually.
Although the application of these four tools was hindered by the evolution on the dismantling site itself, we hope that their intrinsic qualities will make them more widely used in the future.
samedi 29 septembre 2007
FULL CONCRETE RECYCLING
J.R. Costes*, C. Majcherczyk† & I. Peeze Binkhorst‡
*omogine@yahoo.fr
Statistics show that, after potable water, concrete is the most widely consumed product. The enormous quantities involved call for ecological management, which clearly includes recycling. Of all the CO2 released into the atmosphere in 1991, 70 million tons of carbon were attributed to cement manufacturing throughout the world. Recycling cement, however, does not release CO2 since no limestone is added. Concrete is made from about 1 volume of cement for 2 volumes of sand and 3 volumes of aggregates. Recycling only the aggregates is in fact recycling only half the total concrete volume.
We found no references in the literature discussing complete recycling of concrete; research in this area therefore meant tackling a new problem. Basically, the issue was to determine whether the hydration of pure cement is a reversible process. Despite the lack of published work on the subject, the chemistry involved was promising.
We used normal Portland cement (NPC type 55) for these trials. We first prepared six 4 × 4 × 16 cm pure-paste specimens with a water/cement ratio such that a 10 mm dia. Vicat probe penetrated to a depth of 34 mm, indicating a pure paste of standard consistency. The specimens were hardened either at 60°C and 100% relative humidity, or at room temperature. We measured their mechanical properties after 6 days for the autoclaved specimens, and after 28 days for the room-temperature specimens. The specimens were next ground to the required particle size and cured for 30 minutes at 1450°C using standard cement kiln procedures. The resulting clinker was ground to standard cement particle size and the fabrication cycle was repeated to obtain pure-paste specimens. We compared the properties of the recycled specimens with those of the initial reference materials.
Elemental chemical analysis was performed by atomic absorption flame spectrometry after melting with lithium metaborate and acid dissolution. The results obtained for the cement powder before and after reclinkering show that the oxide chemical composition was only slightly modified. More significant differences were observed for the ignition loss at 1000°C and the for sulfur trioxide content; we attribute the differences to the water content of the hydrated materials (water is eliminated during heat treatment at 1000°C) and to sulfate losses during clinkering.
We recorded diffraction diagrams (using a Siemens diffractometer with a rotating sample holder and linear detector) on unhydrated cement powder samples corresponding to the reference powder and to powder obtained from a reclinkered pure hardened paste to compare their mineralogical structure. The spectra revealed similar mineralogical species in both samples. We identified the usual NPC clinker minerals, notably tricalcium silicate (3CaO·SiO2 or “C3S”), dicalcium silicate (2CaO·SiO2 or “C2S”) and tricalcium aluminate (3CaO·Al2O3 or “C3A”). Calcium sulfate dihydrate (gypsum) was not identified in the reclinkered material, however; sulfates were present in a different form—probably non-crystalline since they were undetectable in the X‑ray diffraction spectrum.
The same unhydrated cement specimens were observed and analyzed by SEM in conjunction with an X‑ray diffraction spectrometer. The unhydrated NPC reference powder was examined to determine the morphological characteristics and composition of its constituents. The powder consisted of angular and subangular fragments, smooth and grainy, with the same overall composition as clinker; many of the fragments were smaller than 10 µm. The fragmentation of the clinker grains revealed isolated phases in the powder that were consistent with the stoichiometry for C3S and C2S, and for C3A and C4AF (tetracalcium aluminoferrite). Energy-dispersive electron microprobe analysis confirmed the presence of often contiguous pseudo-hexagonal alite (C3S) crystals associated with rounded crystals often twinned with belite (C2S) and bonded together by an interstitial phase.
The morphology and composition of the reclinkered cement paste were examined in the same way. The morphological examination revealed a large number of grains, most of which were smaller than 10 µm; some of the grains appeared to be broken. The clinker composition was identical, with C2S and C3S associated with the interstitial phases C3A and C4AF. The crystals were generally smaller, possibly because of the cooling rate.
We compared the mechanical properties of specimens fabricated from reclcinkered NPC with those of specimens produced from the initial NPC. Compressive testing (Table I) show that the reclinkered cement material has hydraulic properties and that its compressive strength (128 MPa) exceeds that of the initial pure paste. In other words, the composition and properties of the material obtained from reclinkering a hardened Type 55 NPC paste were comparable to those of the initial cement, and the original hydraulic properties were restored. These properties are related to the presence of anhydrous mineral species that are specific to cement (C2S, C3S, C3A) and cause it to harden in water. Reactivating NCP paste by reclinkering at 1450°C in cement kiln conditions produces a clinker with equivalent or even improved composition and hydraulic properties when compared with the original cement.
Demonstrating that pure hydrated cement can be reclinkered to restore or even improve its properties opened the way toward complete recycling of concrete. We could then address the difficult task of separating the three major components: aggregates, sand and cement.
Fine particles of hydrated cement adhered to the sand and aggregates. Although this was only to be expected, as it constitutes the strength and cohesion of the concrete, it is also the Gordian knot of the separation process: for adequate separation to take place, it must be “undone”.
Pursuing research done in Dutch universities1, the Dutch company KEMA developed an aggregate separation process known as DECO2. We decided this would be a good starting point, and it turned out to be even better than expected. Our study shows that poor results are obtained by attempting to separate the constituents directly, i.e. without preheating the crushed concrete to 700°C as in the DECO process. Applying electrostatic and magnetic separation techniques directly to the concrete also produces mediocre results. The 700°C preheating step in KEMA’s DECO process thus appears to be a suitable—or even indispensable—means of weakening the adherence of the hydrated cement fines to the sand and aggregates.
Our raw material consisted of two concrete samples over 50 years old, one (CS) from Sellafield (UK) and the other (CM) from Marcoule (France). We found that controlled attrition of the crushed concrete down to 2.5 mm was the best way to break up the constituents; forced sieving at 0.125 mm (or a similar process) actually separates the minute particles (15 µm) of hydrated cement from the much larger sand and crushed aggregate particles. This can be done at laboratory scale by forced sieving at 0.125 mm using a flat spatula, and at industrial scale using Deval, Los Angeles or other ball milling equipment.
The forced sieving or controlled attrition step must produce a hydrated cement phase that is as pure as possible. We obtained 60% to 70% pure hydrated cement, the remainder consisting of a mixture of crushed sand and other aggregates. This level of purity requires additives—notably calcium carbonate—to obtain an 85% pure hydrated cement (crude) that can easily be reclinkered. Depending on cost requirements and on the desired objective, we suggest the use of electrostatic or magnetic separation techniques to avoid the need for additives.
Doping the hydrated cement samples HCM and HCS produced crudes that met the criteria for the coefficients and moduli for the fabrication of the type CLC CEM V/A cement we wanted to obtain. The crudes were cured in a simulation kiln, with blast furnace slag and fly ash added after curing, at the Origny Cement Works (Belgium).
The clinkers were ground in the laboratory to a Blaine specific surface area (BSS) of 4000 cm2·g‑1 for two cement samples consisting of 23% Dunkerque blast furnace slag (indispensable for producing CLC), Hornaing fly ash, 49% clinker (CM or CS) and 5% Taverny gypsum to maintain the SO3 content constant at 2.5%. The cement samples were submitted to elemental analysis, particle size analysis, mineralogical constituent determination, specific weight and BSS measurements. The cements were compared with a reference cement (Lumbres V/A 32.5 PM ES CPI – 1999).
Four mortars were prepared from the reclinkered cement and blast furnace additives: two with sand recovered from the CM and CS samples (sand 1 contained two size fractions: 1 [0.15–0.5 mm] and 2 [0.5–2.8 mm] from crushed CM concrete; sand 2 consisted of a single size fraction [0–2.5 mm] from crushed CS concrete), and two others with standard Fontainebleau sand. The actual mortar compositions, mixing water quantities, workability flow rates and bulk densities of the green mortars are summarized in Table II; the 28‑day bending and compressive strength values for the four mortar compositions are indicated in Table III; mortar samples 645, 646 and 647 are shown in Figure 1 after overcompression testing.
The mechanical strength of the two reconstituted CLC cements after 28 days exceeded 50 MPa, allowing them to be classified easily as 42.5. The average 28‑day compressive strength for the mortar prepared from CS cement and Sand 2 from Sellafield was 37.3 MPa. This relatively low figure can be attributed to the single grain size fraction in Sand 2, which prevented optimization. The mortar prepared from CM cement with optimized 1-2 sand (all from Marcoule) performed very well, with an average compressive strength of 61.1 MPa. The tests show that it is preferable to separate the sand into two size fractions and reconstitute them in order to improve the mechanical properties of the mortars.
This laboratory-scale project involving a few kilograms of material demonstrates the feasibility of completely recycling concrete using standard dry industrial procedures: heating, crushing, controlled attrition, and clinkering. These techniques are well known to concrete professionals, and can thus be applied to all types of concrete rubble from the demolition of civil industrial buildings, at a cost near that of new concrete (only a matter of scale). This in turn would make it unnecessary to open new quarries, limit carbon dioxide emissions, and reduce the volume of ground-sterilizing dumped rubble.
1. Bakiewicz, J.L. & Reymer, A.P.S. Separation of Contaminated Concrete, European Report EUR 12562 EN (1990).
2. Cornelissen, H.A.W. Development of a Process for Separating Radioactive Constituents of Concrete, Including Active Pilot-Scale Testing, European Report EUR 16917 EN (1996).
Total recycling of concrete
J.R. Costes*, C. Majcherczyk† & I. Peeze Binkhorst‡
*Commissariat à l’Énergie Atomique (CEA), Rhône Valley Research Center, BP 17171, 30207 Bagnols-sur-Cèze Cedex, France
†CEBTP, 78470 Saint Rémy les Chevreuse, France
‡KEMA, P.O. Box 9035, 6800 ET Arnhem, The Netherlands
Concrete rubble is produced in huge quantities by demolition. Metals and glass are generally recycled industrially, and it would be worthwhile to do the same with concrete. A patient, four-year laboratory project has demonstrated that complete, dry recycling of concrete is possible, based on the finding that hydrated cement can be reclinkered to produce cement of the original quality. Starting with vintage concrete that was crushed and heated to 700°C for four hours, we successfully separated the hydrated cement from the sand and aggregates by attrition, obtaining 60% to 70% pure hydrated cement. Further separation can be obtained by an electrostatic or magnetic process yielding 85% purity. The resulting hydrated cement can be reclinkered with or without additives to produce high-quality Portland or CLC cement (a mixture of Portland cement and blast furnace slag cement). We obtained mortar of excellent quality using the constituents separated in this way. These results plead for ecologically sound management of concrete on a much broader scale.
Statistics show that, after potable water, concrete is the most widely consumed product. The enormous quantities involved call for ecological management, which clearly includes recycling. Of all the CO2 released into the atmosphere in 1991, 70 million tons of carbon were attributed to cement manufacturing throughout the world. Recycling cement, however, does not release CO2 since no limestone is added. Concrete is made from about 1 volume of cement for 2 volumes of sand and 3 volumes of aggregates. Recycling only the aggregates is in fact recycling only half the total concrete volume.
We found no references in the literature discussing complete recycling of concrete; research in this area therefore meant tackling a new problem. Basically, the issue was to determine whether the hydration of pure cement is a reversible process. Despite the lack of published work on the subject, the chemistry involved was promising.
We used normal Portland cement (NPC type 55) for these trials. We first prepared six 4 × 4 × 16 cm pure-paste specimens with a water/cement ratio such that a 10 mm dia. Vicat probe penetrated to a depth of 34 mm, indicating a pure paste of standard consistency. The specimens were hardened either at 60°C and 100% relative humidity, or at room temperature. We measured their mechanical properties after 6 days for the autoclaved specimens, and after 28 days for the room-temperature specimens. The specimens were next ground to the required particle size and cured for 30 minutes at 1450°C using standard cement kiln procedures. The resulting clinker was ground to standard cement particle size and the fabrication cycle was repeated to obtain pure-paste specimens. We compared the properties of the recycled specimens with those of the initial reference materials.
Elemental chemical analysis was performed by atomic absorption flame spectrometry after melting with lithium metaborate and acid dissolution. The results obtained for the cement powder before and after reclinkering show that the oxide chemical composition was only slightly modified. More significant differences were observed for the ignition loss at 1000°C and the for sulfur trioxide content; we attribute the differences to the water content of the hydrated materials (water is eliminated during heat treatment at 1000°C) and to sulfate losses during clinkering.
We recorded diffraction diagrams (using a Siemens diffractometer with a rotating sample holder and linear detector) on unhydrated cement powder samples corresponding to the reference powder and to powder obtained from a reclinkered pure hardened paste to compare their mineralogical structure. The spectra revealed similar mineralogical species in both samples. We identified the usual NPC clinker minerals, notably tricalcium silicate (3CaO·SiO2 or “C3S”), dicalcium silicate (2CaO·SiO2 or “C2S”) and tricalcium aluminate (3CaO·Al2O3 or “C3A”). Calcium sulfate dihydrate (gypsum) was not identified in the reclinkered material, however; sulfates were present in a different form—probably non-crystalline since they were undetectable in the X‑ray diffraction spectrum.
The same unhydrated cement specimens were observed and analyzed by SEM in conjunction with an X‑ray diffraction spectrometer. The unhydrated NPC reference powder was examined to determine the morphological characteristics and composition of its constituents. The powder consisted of angular and subangular fragments, smooth and grainy, with the same overall composition as clinker; many of the fragments were smaller than 10 µm. The fragmentation of the clinker grains revealed isolated phases in the powder that were consistent with the stoichiometry for C3S and C2S, and for C3A and C4AF (tetracalcium aluminoferrite). Energy-dispersive electron microprobe analysis confirmed the presence of often contiguous pseudo-hexagonal alite (C3S) crystals associated with rounded crystals often twinned with belite (C2S) and bonded together by an interstitial phase.
The morphology and composition of the reclinkered cement paste were examined in the same way. The morphological examination revealed a large number of grains, most of which were smaller than 10 µm; some of the grains appeared to be broken. The clinker composition was identical, with C2S and C3S associated with the interstitial phases C3A and C4AF. The crystals were generally smaller, possibly because of the cooling rate.
We compared the mechanical properties of specimens fabricated from reclcinkered NPC with those of specimens produced from the initial NPC. Compressive testing (Table I) show that the reclinkered cement material has hydraulic properties and that its compressive strength (128 MPa) exceeds that of the initial pure paste. In other words, the composition and properties of the material obtained from reclinkering a hardened Type 55 NPC paste were comparable to those of the initial cement, and the original hydraulic properties were restored. These properties are related to the presence of anhydrous mineral species that are specific to cement (C2S, C3S, C3A) and cause it to harden in water. Reactivating NCP paste by reclinkering at 1450°C in cement kiln conditions produces a clinker with equivalent or even improved composition and hydraulic properties when compared with the original cement.
Demonstrating that pure hydrated cement can be reclinkered to restore or even improve its properties opened the way toward complete recycling of concrete. We could then address the difficult task of separating the three major components: aggregates, sand and cement.
Fine particles of hydrated cement adhered to the sand and aggregates. Although this was only to be expected, as it constitutes the strength and cohesion of the concrete, it is also the Gordian knot of the separation process: for adequate separation to take place, it must be “undone”.
Pursuing research done in Dutch universities1, the Dutch company KEMA developed an aggregate separation process known as DECO2. We decided this would be a good starting point, and it turned out to be even better than expected. Our study shows that poor results are obtained by attempting to separate the constituents directly, i.e. without preheating the crushed concrete to 700°C as in the DECO process. Applying electrostatic and magnetic separation techniques directly to the concrete also produces mediocre results. The 700°C preheating step in KEMA’s DECO process thus appears to be a suitable—or even indispensable—means of weakening the adherence of the hydrated cement fines to the sand and aggregates.
Our raw material consisted of two concrete samples over 50 years old, one (CS) from Sellafield (UK) and the other (CM) from Marcoule (France). We found that controlled attrition of the crushed concrete down to 2.5 mm was the best way to break up the constituents; forced sieving at 0.125 mm (or a similar process) actually separates the minute particles (15 µm) of hydrated cement from the much larger sand and crushed aggregate particles. This can be done at laboratory scale by forced sieving at 0.125 mm using a flat spatula, and at industrial scale using Deval, Los Angeles or other ball milling equipment.
The forced sieving or controlled attrition step must produce a hydrated cement phase that is as pure as possible. We obtained 60% to 70% pure hydrated cement, the remainder consisting of a mixture of crushed sand and other aggregates. This level of purity requires additives—notably calcium carbonate—to obtain an 85% pure hydrated cement (crude) that can easily be reclinkered. Depending on cost requirements and on the desired objective, we suggest the use of electrostatic or magnetic separation techniques to avoid the need for additives.
Doping the hydrated cement samples HCM and HCS produced crudes that met the criteria for the coefficients and moduli for the fabrication of the type CLC CEM V/A cement we wanted to obtain. The crudes were cured in a simulation kiln, with blast furnace slag and fly ash added after curing, at the Origny Cement Works (Belgium).
The clinkers were ground in the laboratory to a Blaine specific surface area (BSS) of 4000 cm2·g‑1 for two cement samples consisting of 23% Dunkerque blast furnace slag (indispensable for producing CLC), Hornaing fly ash, 49% clinker (CM or CS) and 5% Taverny gypsum to maintain the SO3 content constant at 2.5%. The cement samples were submitted to elemental analysis, particle size analysis, mineralogical constituent determination, specific weight and BSS measurements. The cements were compared with a reference cement (Lumbres V/A 32.5 PM ES CPI – 1999).
Four mortars were prepared from the reclinkered cement and blast furnace additives: two with sand recovered from the CM and CS samples (sand 1 contained two size fractions: 1 [0.15–0.5 mm] and 2 [0.5–2.8 mm] from crushed CM concrete; sand 2 consisted of a single size fraction [0–2.5 mm] from crushed CS concrete), and two others with standard Fontainebleau sand. The actual mortar compositions, mixing water quantities, workability flow rates and bulk densities of the green mortars are summarized in Table II; the 28‑day bending and compressive strength values for the four mortar compositions are indicated in Table III; mortar samples 645, 646 and 647 are shown in Figure 1 after overcompression testing.
The mechanical strength of the two reconstituted CLC cements after 28 days exceeded 50 MPa, allowing them to be classified easily as 42.5. The average 28‑day compressive strength for the mortar prepared from CS cement and Sand 2 from Sellafield was 37.3 MPa. This relatively low figure can be attributed to the single grain size fraction in Sand 2, which prevented optimization. The mortar prepared from CM cement with optimized 1-2 sand (all from Marcoule) performed very well, with an average compressive strength of 61.1 MPa. The tests show that it is preferable to separate the sand into two size fractions and reconstitute them in order to improve the mechanical properties of the mortars.
This laboratory-scale project involving a few kilograms of material demonstrates the feasibility of completely recycling concrete using standard dry industrial procedures: heating, crushing, controlled attrition, and clinkering. These techniques are well known to concrete professionals, and can thus be applied to all types of concrete rubble from the demolition of civil industrial buildings, at a cost near that of new concrete (only a matter of scale). This in turn would make it unnecessary to open new quarries, limit carbon dioxide emissions, and reduce the volume of ground-sterilizing dumped rubble.
1. Bakiewicz, J.L. & Reymer, A.P.S. Separation of Contaminated Concrete, European Report EUR 12562 EN (1990).
2. Cornelissen, H.A.W. Development of a Process for Separating Radioactive Constituents of Concrete, Including Active Pilot-Scale Testing, European Report EUR 16917 EN (1996).
<> Style tag for the Acknowledgements.
Correspondence should be addressed to J.R.C. (e-mail: mailto:omogine@yahoo.fr.
Table I. Compression test results for ordinary Portland cement specimens before and after reclinkering
Table II. Reconstituted mortar compositions (kg·m‑3)
Figure 1: Mortar samples made with recycled concrete: “Green concrete” after overcompression testing.
<> Style tag for the Acknowledgements.
Correspondence should be addressed to J.R.C. (e-mail: mailto:omogine@yahoo.fr.